Techniques to take atmospheric CO2 and turn it into a fuel provide a climate-friendly alternative to exploiting fossil fuels—they may releases CO2 back into the air when burned, but there's no net change. This includes biofuels crops, but can extend to industrial processes that directly involve CO2. As processes that capture CO2 from ambient air become more economical, so will the potential value of that CO2 as a resource for fuels.
There are a few ways to go about making fuel, but all require considerable energy because CO2 is a stable molecule—reversing the combustion reaction to make a new fuel doesn’t happen for free. But there's an additional challenge: designing a process tuned to produce the exact type of fuel you want.
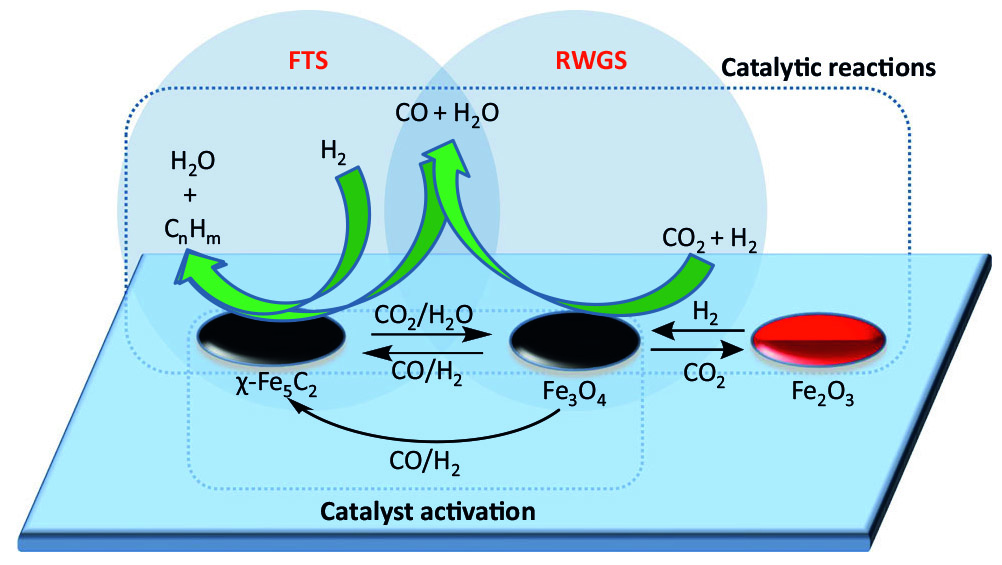
One way to do that is with a catalyst—a substance that guides the chemical reactions without being consumed by them. With the help of one catalyst, captured CO2 plus hydrogen gas might primarily be turned into methane; a different catalyst might shift the primary product towards the larger molecules of liquid fuels.
A new study led by Benzhen Yao at the University of Oxford describes a new catalyst that specializes in driving the production of the long-chain hydrocarbons used for jet fuel.
The numbers
That catalyst is an iron-manganese-potassium material—nothing too exotic. In tests, only five percent of the converted CO2 ended up as carbon monoxide and 10 percent ended up as methane, while nearly half turned into long-chain hydrocarbons in the jet fuel range (8-16 carbons). Compared to other catalysts tested in previous studies, that’s a much better output in the jet fuel range. And of the 2-4 carbon hydrocarbons produced, it favors alkenes over alkanes, which means a more carbon-carbon bonds and fewer carbon-hydrogen bonds. The reaction made five times as much propylene as propane, for example. Those are useful raw materials for things like plastics.


 Loading comments...
Loading comments...
